“What I love about science is that as you learn, you don’t really get answers. You just get better questions.” – John Green
Project description:
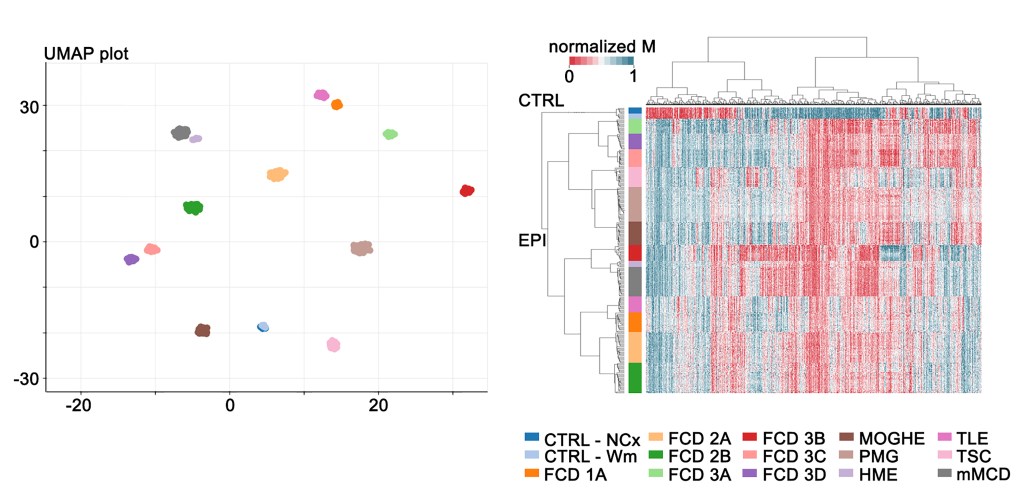
From Mechanism to Biomarker: DNA Methylation in Focal Epilepsy
Focal epilepsies, often characterized by structural brain lesions such as hippocampal sclerosis, malformations of cortical development (MCD), or glioneuronal tumors, represent a significant subset of epilepsy cases, are frequently drug-resistant, and have an increased risk of premature mortality and sudden unexpected death.
Surgical resection of structural brain lesions offers hope for seizure control, but success rates are limited, with 50-80% of patients experiencing favorable outcomes. Identifying factors that contribute to a successful surgical outcome and predicting surgery refractoriness are crucial unmet clinical needs in this field.
Drawing inspiration from the realm of cancer research, where specific epigenetic and genetic profiles have revolutionized disease prognosis, our lab aims to explore the diagnostic and predictive value of DNA methylation in focal epilepsies amenable to epilepsy surgery.
Our research focuses on understanding the role of epigenetics in the pathogenesis of focal epilepsy associated with structural brain lesions and developing diagnostic tools, utilizing genomic DNA methylation patterns obtained, e.g., from brain tissue and peripheral blood samples.
Join us in deciphering the mysteries of epilepsy and advancing the field of molecular neuropathology, one methylation pattern at a time.
Who:
Mitali Katoch
Funding:
Related Content:
Project description:
How Epilepsy Medications Affect Brain Development Across Generations
Epilepsy affects around 50 million people worldwide, including many women of childbearing age. In the U.S. alone, over half a million women with epilepsy continue treatment during pregnancy and breastfeeding. But this is also a critical time for a baby’s brain to develop—and certain medications taken by the mother may affect this process.
Some anti-seizure drugs (ASDs), like valproic acid (VPA), are known to increase the risk of brain malformations or mental health problems in children exposed before birth. As a result, prescription guidelines for these drugs have been updated. However, we still don’t know enough about the safety of newer medications during pregnancy.
In collaboration with the group of Christophe Bernard, Marseille, France, this project investigates how ASDs affect brain development and function across generations.
Project description:
Decoding the Matrix: The Role of Matrix Mechanics in Neuronal Synchronization
The brain’s extracellular matrix (ECM), comprising fibrous proteins, glucosaminoglycans, proteoglycans, and glycoproteins, plays a crucial role in shaping brain structure, excitability, and overall function. During brain development, the ECM undergoes substantial changes, impacting cellular behaviors such as proliferation, adhesion, migration, differentiation, and cell death.
Despite advances in understanding the ECM’s role in brain development and disease, the impact of mechanical cues on neuronal activity remains unclear. Abnormal ECM conditions are linked to genetic and acquired epilepsy syndromes, prompting exploration for therapeutic interventions. This project seeks to unveil the intricate relationship between matrix mechanics and neuronal synchronization, contributing to a mechanistic understanding of cellular and circuit function. Our goal is to lay the groundwork for innovative, informed treatment approaches in epilepsy. Join us on this journey to uncover the secrets of the neuronal matrix interface.

Project description:
Unlocking the Secrets of CNS Scarring
Central nervous system (CNS) injuries, such as those occurring in the brain and spinal cord, often lead to the formation of inhibitory scar tissue in mammals, hindering axon regeneration. In striking contrast, zebrafish possess the remarkable ability to regenerate axons in response to CNS injury. The molecular basis underlying these divergent outcomes remains elusive.
This project delves into the complexities of CNS scarring focussing on Small Leucine-Rich Proteoglycans (SLRPs). They are a group of proteins abundant in human and rodent CNS lesions but notably absent in zebrafish. Uncovering the mysteries behind varied outcomes of CNS injury in different species, will offer valuable insights for therapeutic interventions in individuals affected by traumatic brain or spinal cord injuries in the future.

Project description:
Who is in charge? Circadian rhythms in the epilepsies
Circadian rhythms are the body’s internal clock, regulating a variety of physiological processes over a roughly 24-hour cycle. These rhythms are controlled by the suprachiasmatic nucleus in the hypothalamus and influence sleep, hormone release, and cellular metabolism. Disruptions to circadian rhythms can impair brain function and have been linked to various neurological disorders, including epilepsy. Research indicates that circadian rhythms may modulate seizure susceptibility and neuronal excitability, with some types of epilepsy showing a clear diurnal pattern of seizure occurrence.
The Kobow Lab in collaboration with the the ChronoEpilepsyLab (@chrono_lab) under the supervision of Prof. Cristina R. Reschke explores the molecular and cellular underpinnings of the circadian rhythm in epilpesy.
Who:
Hendrik Zimmermann
Funding:
IZKF
Related Content:
The Kobow Lab
Universitätsklinikum Erlangen
Dept. of Neuropathology
Schwabachanlage 6,
91054 Erlangen, Germany
info@kobowlab.org
+49 (9131) 85-34782
Präsentiert von WordPress